Background
Follicular lymphoma (FL) is an indolent disease with favorable outcome in patients (pt) treated with antibody-based immunochemotherapy. However, ca. 15% of the pt population experience disease relapse within 24 months or transformation to a more aggressive disease, representing a population with high unmet medical need. Available clinicogenetic risk scores show suboptimal performance for predicting early disease progression (Jurinovic, Blood 2016). Here we evaluated ctDNA at screening as a novel tool to identify high risk pt populations.
Material and Methods
Pre-treatment plasma samples from 221 selected pts diagnosed with advanced stage or bulky FL in need of systematic treatment from the GALLIUM trial (NCT 01332968) were analyzed. Pts were selected to be evenly distributed among chemo/antibody groups (50 Rituximab (R) -Bendamustine (Benda), 63 R-CHOP/CVP, 50 Gazyva (G) -Benda, 58 G-CHOP/CVP). Within each treatment group, 25 to 28 cases with good responses (progression-free survival (PFS) > 5 years) and 25 to 31 cases with early progression or transformation to an aggressive disease (median PFS: 352 days and 91.6% of these cases progressed within 24 months) were selected. Targeted NGS was performed using a modified version of the AVENIO ctDNA analysis workflow (Roche; Research Use Only) based on the previously described CAPP-Seq workflow (Kurtz, J Clin Oncol 2018). The targeted NGS panel was designed to cover ~314 kb in or near 466 genes associated with NHL. Potential germline derived variants were filtered out using a publicly available database and panel specific blacklist filtering; additionally, variants with allelic frequency (AF) >20% were excluded. ctDNA was quantified as mutant molecules per milliliter-of-plasma (MMPM) based on single nucleotide variants (SNV) in the sequenced genomic regions. The association of PFS with this classifier was evaluated by Kaplan-Meier methods and Cox proportional hazards model, and an optimal cut-off for MMPM high vs low was determined by using maximally selected rank statistics, validated by 10-fold cross validation.
Results
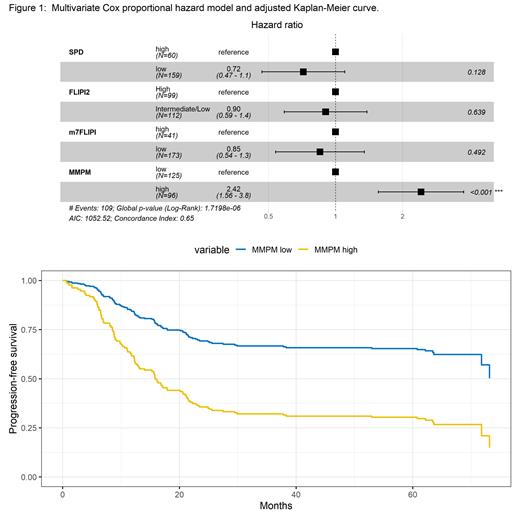
We detected a median of 107 (range 0-554) SNV at a median deduplicated depth of 3766x. A strong correlation between ctDNA at screening, measured as MMPM, and clinical prognostic markers was observed; higher levels of MMPM were associated with larger sum of the product of the diameters (SPD) (Mann-Whitney-U (MWU) P < 0.0001), higher FLIPI2 scores (MWU P < 0.0001) and m7-FLIPI (MWU P = 0.0001). Pts with long PFS had a significantly (MWU P < 0.0001) lower median MMPM (59.9) than those who progressed early (276.5). Using an optimal cut-off to predict pts at risk of early progression, MMPM high cases (n=96) showed significantly more total SNVs compared to MMPM low cases (n=125) (MWU P < 0.0001). MMPM high vs low was a stronger marker for PFS (cross-validated log-rank P < 0.0001; HR [95% CI] = 2.4 [1.6,3.5]) versus FLIPI2 (log-rank P = 0.0063; HR [95% CI] = 1.7 [1.2,2.4]) or tumor burden measured as SPD (log-rank P = 0.0009; HR [95% CI] = 1.9 [1.3,2.8]). In a multivariate Cox model, the association of high MMPM and worse PFS was still significant after correction for FLIPI2, SPD and m7-FLIPI, indicating that MMPM has an independent prognostic value (log-rank P < 0.0001; HR [95% CI] = 2.42 [1.6,3.8]) (Figure 1). Consistent with previous reports (Jurinovic, ASH 2021), also in this selected cohort m7-FLIPI was prognostic for CHOP/CVP (log-rank P = 0.0061; HR [95% CI] = 2.2 [1.2,3.8]) but not for Benda (log-rank P = 0.2274; HR [95% CI] = 1.5 [0.76,3.0]). Notably, the prognostic value of ctDNA at screening using MMPM was observed for both CHOP/CVP (log-rank P < 0.0001; HR [95% CI] = 2.9 [1.7,5.0]), and Benda (log-rank P < 0.0001; HR [95% CI] = 2.9 [1.7,5.1]).
Conclusion
Measurements of ctDNA at screening showed promising results as a biomarker to predict high risk populations for early treatment failure in untreated FL. MMPM at baseline, quantified using the AVENIO NHL assay, showed an independent prognostic value compared to FLIPI 2, m7-FLIPI and SPD. Notably, while known clinicogenetic risk scores had a prognostic value limited to CHOP/CVP regimen (Jurinovic, ASH 2019, Bolen, Blood 2021), MMPM prognostic value was maintained on CHOP/CVP and Benda chemotherapy. Overall, these initial results warrant additional analysis to investigate the value of the ctDNA as a treatment agnostic stratification tool for clinical research.
Disclosures
Bottos:F. Hoffmann La Roche Ltd: Current Employment, Current holder of stock options in a privately-held company. Woestmann:Employee of Signature Diagnostics GmbH: Current Employment. Bolen:Genentech, Inc.: Current Employment; F Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Shin:Roche Molecular Solutions: Current Employment. Bogard:Roche Sequencing Solutions: Current Employment; F. Hoffmann-La Roche Ltd, Basel, Switzerland;: Current equity holder in private company, Current holder of stock options in a privately-held company. Tabari:Freenome: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Roche Sequencing Solutions: Ended employment in the past 24 months. Davies:Genmab: Consultancy, Honoraria; Kite/Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellcentric: Research Funding; MSD: Research Funding; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grant, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Sobi: Consultancy; Incyte: Consultancy; Abbvie: Consultancy, Honoraria. Knapp:F. Hoffmann-La Roche Ltd: Current Employment. Nielsen:F. Hoffmann-La Roche Ltd,: Current Employment, Current equity holder in private company, Divested equity in a private or publicly-traded company in the past 24 months. Penuel:Genentech, Inc. / F. Hoffman-La Roche Ltd: Current Employment, Current holder of stock options in a privately-held company.